Medical device lifecycle: what to pay attention to at each stage
As a medical device producer, you are responsible for the safety and effectiveness of your medical device throughout all stages of its lifecycle. You must ensure that the product remains compliant with all the regulatory requirements and that you follow the necessary processes.
In this article, prepared by Sara Juszczyk, Quality and Regulatory Affairs Manager at Spyrosoft, we discuss a medical device lifecycle with a focus on the producer’s responsibilities at each stage – from the initial design to product end of life.
What are the stages of the medical device lifecycle?
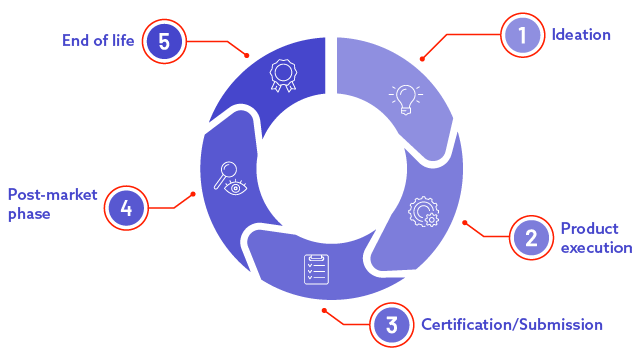
Ideation
The main goal of this stage is to determine whether the product will be considered a medical device or not according to the applicable regulations, like MDR 2017/745 in the EU or FDA Title 21 in the USA. That includes determining the intended use of a device and identifying the main risks related to the device use.
When a medical device’s status is confirmed, the regulatory strategy needs to be drafted. Regulatory would cooperate with the business to understand the business case and business objectives behind introducing the product to the market. The strategy includes the analysis and justification for product classification, which determines specific subsequent requirements applicable to the product. However, the most important aspect of the strategy would be a recommendation for a solution or a set of solutions to streamline the introduction to the market considering the markets of interest.
For example, if your company would like to enter the EU, US and UK markets, which country should you prepare for first? Would it be possible to enter all the markets simultaneously? What would be the impact of the new MDR and Brexit on the company’s business case? What would be the additional specific requirements, like clinical trials, cybersecurity, human factor testing and so forth? Lastly, what would be the timeline, potential risks to the project, or setbacks when considering different routes and scenarios?
The earlier the regulatory strategy is prepared, the better for a medical device manufacturer. Often, at an early stage, it is possible to mitigate risks to the project and secure an effective market introduction by slightly changing the way the product would be marketed (e.g. accessories to be sold separately, or alternatively – as a set, or introducing more secure architecture to software before the code was even created). Identifying those potential hiccups at a later stage could definitely be more expensive and result in a higher risk of complications and delays along the way.
Product Execution
A crucial step at this stage is to implement a Quality Management System unless it has already been implemented. Additionally, when developing a medical device containing software, or software as a medical device, procedures according to IEC 62304:2006 need to be followed. Medical software (irrespective of whether it is embedded or SaMD) must follow the versioning process as it’s being developed. All the identified bugs must be described and documented. The development process itself also has to be documented with all the details included, like the configuration and integration of specific modules. Design process documentation is very detailed. Therefore, it requires in-depth knowledge and significant experience.
During product development, a prototype will be created. A prototype, depending on its characteristics, will undergo a series of tests. The first step would be the verification – checking if the device was designed according to the plans and user input. That includes specific performance testing. Secondly, validation needs to be performed. At this point, things may get tricky. As a manufacturer, you need to prove that the device fulfils its intended medical use, according to the expectations.
As a result of this phase, a design dossier should be created, documenting a working, safe and effective device. Product documentation will be an input to the next phase.
Our specialists can support you in preparing the necessary documentation, ensuring compliance with all the requirements and QMS implementation. Find out more details on our healthcare offering website.
Our experts will help you manage your medical software’s lifecycle
Find out moreCertification/Submission
This phase includes the final preparation for the long-awaited introduction of the product to the market. As soon as the product development and testing are complete, the documentation is prepared, and the QMS is implemented, it’s time to prepare additional documentation for the actual submission or certification process.
During this stage, typically, there are questions from the reviewer or the regulatory authority that our consultants also help to address and provide guidance on. Similarly, they will assist in a case when any nonconformities are identified.
Post-market phase
Once you have obtained permission to market your product, you can begin selling it. During the post-market phase, usually the longest stage of the medical device’s lifecycle, post-market surveillance activities must be performed, depending on device classification and the subject market. In the EU, that would be periodical reporting and PMCF (Post-Market Clinical Follow-up).
These activities aim to provide data and evidence regarding a medical device’s safety and performance in the real world. That includes addressing and reporting any possible adverse events, continuous monitoring, and collecting feedback from the market.
Apart from monitoring the safety of the device, naturally, as a manufacturer, you would like to further develop your products. That means upgrading technologies and adding new features or models to existing medical devices. However, assessing any planned modifications from a regulatory perspective is critical. A seemingly minor change may require a completely new, separate submission. Especially regarding software, any changes need to be properly tested and documented before the implementation. A special focus should be put here because historically, the biggest number of adverse events related to software malfunction was caused by changes to the software.
Finding a balance between business needs and patient safety is crucial. It is vital to work out the most effective solution to satisfy the needs of both sides. That is a challenge our experts in regulatory compliance can help you with. We’ll guide you through the post-market phase and suggest good practices and solutions that may help you conduct all the activities more effectively and, as a result, develop your business.
End of life
The last phase is the withdrawal of a medical device from the market as the result of a business decision.
Choose an experienced partner to help you manage your medical software’s lifecycle
The most crucial aspect of successful medical device lifecycle management from the regulatory perspective is understanding the business side on the one hand and on the other – the in-depth knowledge of the global requirements, mapping out the processes and implementing the best practices. Ad-hoc decisions without considering the regulatory aspect may result in costly consequences, like adding regulatory obligations and prolonging project timelines.
Choosing the right partner who understands the business side of the projects and has working experience with medical process standards, such as ISO 13485 and IEC 62304 is crucial to ensure the smooth launch of your product and its further development. See the details of our offer and contact us to discuss your needs.
About the author
RECOMMENDED ARTICLES
Contact us